GORE® VIABIL® Biliary Endoprosthesis
Fully Covered Metal Stent
GORE® VIABIL® Biliary Endoprosthesis
Maximize control. Minimize migration.
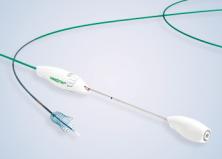
Through its partnership with W. L. Gore & Associates, Inc., Olympus brings a fully covered self-expanding metal stent to healthcare providers caring for patients with malignant biliary obstruction. The GORE® VIABIL® Biliary Endoprosthesis offers a solution for the treatment of malignant biliary strictures by addressing key challenges in biliary stenting and enabling the restoration of bile flow. This device features unique anti-migration fins proven to reduce the risk of reinterventions related to migration†,1, and a non-foreshortening* design that enables precise placement. Its highly conformable construction allows it to adapt to various bile duct anatomies; and the preferred4 balance of radial and axial forces provides the fit and flexibility to help minimize migration.§,4,5
Indications for Use
The GORE® VIABIL® Short Wire Biliary Endoprosthesis is indicated for palliation of malignant strictures in the biliary tree.
Contraindications
The GORE® VIABIL® Short Wire Biliary Endoprosthesis is contraindicated for:
- ALL CARDIOVASCULAR APPLICATIONS
- Ducts less than 5.5 mm in diameter or greater than 9 mm in diameter
Key Benefits
- Anti-migration fins: Atraumatic‡, fully covered anchoring fins securely hold the device within the duct to minimize the risk of migration.*1
- Durable, non-porous Fluorinated Ethylene Propylene (FEP)/expanded Polytetrafluoroethylene (ePTFE) liner: Prevents tissue ingrowth, promotes conformability and supports high primary patency rates.2,3
- Single, continuous wind of Nitinol wire: Designed for conformability and allows for the preferred4 combination of axial and radial forces, naturally following tortuous anatomy.4,5
- Non-foreshortening design* of the endoprosthesis enables accurate deployment positioning.
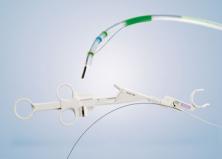
- Precise placement made easy: Exclusive pull line deployment, radiopaque markers and non-foreshortening* stent design enable accurate delivery.
- Soft, curved tapered tip: Facilitates device delivery.
- Optional transmural drainge holes: Intended to allow for endoprosthesis placement across a branch duct under appropriate anatomical circumstances.
- Short wire delivery system.
- O'Neill F, Stevenson A, Swarup VP. Fully covered self-expanding metal stents for endoscopic retrograde cholangiopancreatography and percutaneous cholangiography in the management of malignant biliary obstruction: a U.S. cost-consequence analysis. Presented at DDW 2022 Digestive Disease Week; May 21-24, 2022; San Diego, CA. Gastrointestinal Endoscopy 2022;95(6)Supplement:AB293.
- Krokidis M, Fanelli F, Orgera G, Tsetis D, Mouzas I, Bezzi M, Kouroumalis E, Pasariello R, Hatzidakis A. Percutaneous palliation of pancreatic head cancer: randomized comparison of ePTFE/FEP-covered versus uncovered nitinol biliary stents. Cardiovascular & Interventional Radiology. 2011;34(2):352-361.
- Krokidis M, Fanelli F, Orgera G, Bezzi M, Passariello R, Hatzidakis A. Percutaneous treatment of malignant jaundice due to extrahepatic cholangiocarcinoma: covered Viabil stent versus uncovered Wallstents. Cardiovascular & Interventional Radiology 2010;33(1):97-106.
- Isayama H, Nakai Y, Toyokawa Y, et al. Measurement of radial and axial forces of biliary self-expandable metallic stents. Gastrointestinal Endoscopy 2009;70(1):37-44.
- Isayama H, Mukai T, Itoi T, et al. Comparison of partially covered nitinol stents with partially covered stainless stents as a historical control in a multicenter study of distal malignant biliary obstruction: the WATCH study. Gastrointestinal Endoscopy 2012;76(1):84-92.
* If deployed as instructed, the endoprosthesis will not appreciably foreshorten.
† Data on file 2025; W. L. Gore & Associates, Inc; Flagstaff, AZ.
‡ Data on file 2024; W. L. Gore & Associates, Inc; Flagstaff, AZ.
§ Benchtop evaluations are intended to demonstrate relative physical characteristics and may not correlate to clinical results.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warning, precautions and contraindications for the markets where this product is available. 
Products listed may not be available in all markets.
GORE, VIABIL and designs are trademarks of W. L. Gore & Associates.
GORE® VIABIL® Biliary Endoprosthesis is manufactured by W. L. Gore & Associates, Inc and distributed by Olympus America, Inc.
Complications associated with the use of the GORE® VIABIL® Short Wire Biliary Endoprosthesis may include complications associated with the use of other biliary endoprostheses. These complications include (in alphabetical order) allergic reaction, bleeding due to vascular erosion, endoprosthesis fracture, endoprosthesis migration, endoprosthesis misplacement, endoprosthesis occlusion, and obstruction of branch ducts or the bowel. Please consult the Instructions for Use for more information.
Product Support
| Model Number | Endoprosthesis Diameter (mm) | Endoprosthesis Length (cm) | Delivery Catheter Working Length (cm) | Transmural Drainage Holes Segment Length (cm) | Catheter Diameter (Fr) |
| VSWVN0804 | 8 | 4 | 200 | - | 8.5 |
| VSWVN0806 | 8 | 6 | 200 | - | 8.5 |
| VSWVN0808 | 8 | 8 | 200 | - | 8.5 |
| VSWVN0810 | 8 | 10 | 200 | - | 8.5 |
| VSWVN1004 | 10 | 4 | 200 | - | 8.5 |
| VSWVN1006 | 10 | 6 | 200 | - | 8.5 |
| VSWVN1008 | 10 | 8 | 200 | - | 8.5 |
| VSWVN1010 | 10 | 10 | 200 | - | 8.5 |
| VSWVH0806 | 8 | 6 | 200 | 2 | 8.5 |
| VSWVH0808 | 8 | 8 | 200 | 2 | 8.5 |
| VSWVH0810 | 8 | 10 | 200 | 2 | 8.5 |
| VSWVH1006 | 10 | 6 | 200 | 2 | 8.5 |
| VSWVH1008 | 10 | 8 | 200 | 2 | 8.5 |
| VSWVH1010 | 10 | 10 | 200 | 2 | 8.5 |
Olympus Training & Proper Use
Olympus Continuum, is a comprehensive platform of education and training experiences led by healthcare experts from around the world. Learning opportunities include hands-on courses, online learning, lectures and workshops, peer-to-peer training, accredited continuing education, and on-demand learning.
For more information: Olympus Continuum Video