The Need for Early
Colorectal Cancer Diagnosis
Across the globe, there are over 5,000 new patients diagnosed with colorectal cancer (CRC) every day and more than 2,500 CRC-related deaths.1
Challenges in colonoscopy: Polyp detection
Up to 26% of adenomas are missed during colonoscopies.2
Notably, each 1% increase in adenoma detection rate (ADR) correlates to a 3% decrease in the risk of cancer incidence.3
In recognition of this challenge, the most recent guidelines from ASGE/ACG increased the expectation for ADR performance and also introduced a 6% sessile serrated lesion (SSL) detection rate as a quality indicator.4
5k+
new patients diagnosed with CRC each day1
2.5k+
patients' lives lost to CRC each day1
Connecting AI-driven endoscopy
solutions with established
Olympus® equipment
Olympus introduces the OLYSENSE™ Platform, a cloud-based, integrated and scalable suite of connected apps and solutions that create a sophisticated, simple, and scalable intelligent endoscopy system.
A key component of the OLYSENSE Platform is its CAD/AI portfolio, which includes the CADDIE™ medical device software.
The OLYSENSE™ Platform is part of the EVIS X1™ endoscopy ecosystem of intelligent endoscopy solutions.

CADDIE medical device software leverages cloud-based AI to aid detection of high-risk and hard-to-detect lesions.5
CADDIE software’s AI algorithm training data includes an enriched data set of clinically significant polyps, such as large polyps and SSLs.6
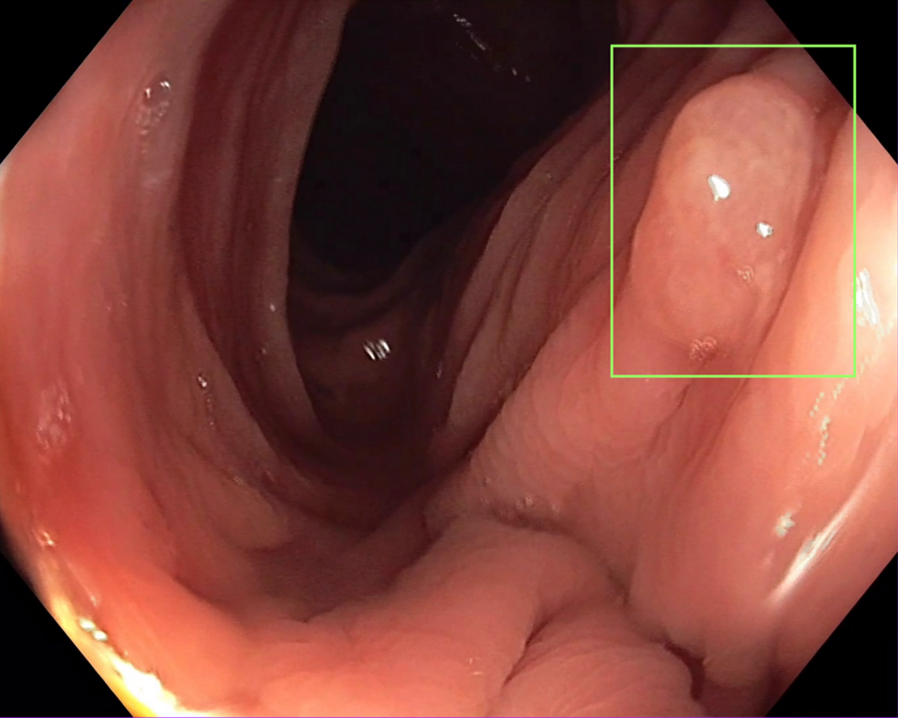
Polyp detection
Image captured using CF-H190L scope.
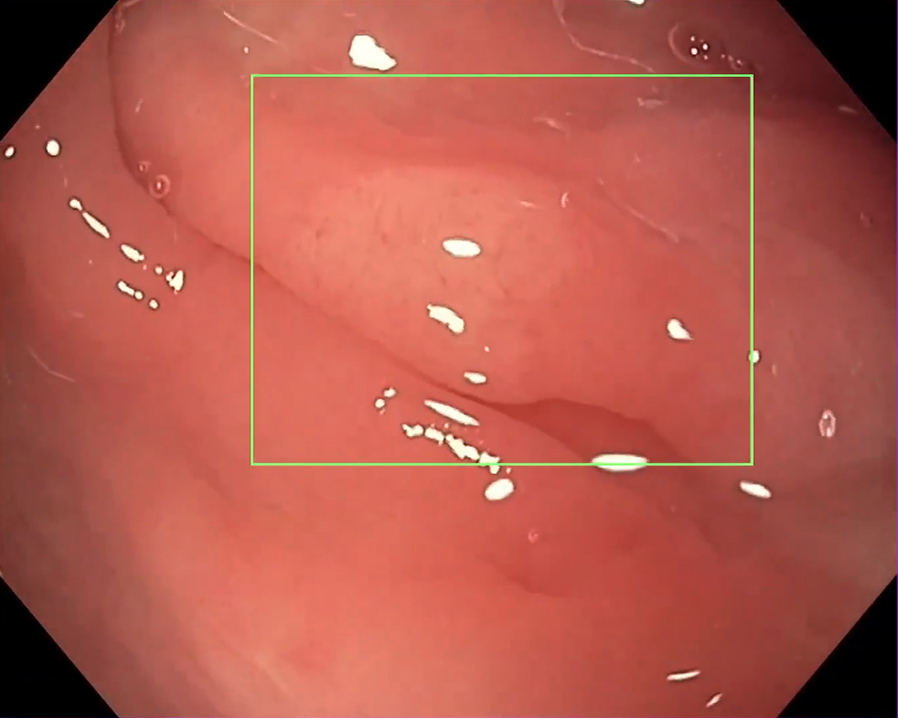
High-risk and hard-to-detect lesions
Image captured using CF-H190I scope.
Find out more about the EVIS X1
endoscopy ecosystem, and the
power of intelligent endoscopy today!
Leveraging AI to Improve Lower GI Endoscopy
Data behind the polyp detection algorithm
Clinically significant polyps
CADDIE software’s AI polyp detection algorithm was developed using a robust dataset of 162,207 image frames from 906 patients, including large polyps and SSLs.6
Clinical accuracy and performance
When using CADDIE software in a multi-center randomized controlled trial conducted across eight European hospitals, endoscopists experience a 7.3% absolute increase in ADR.5

High-risk and hard-to-detect lesions
Combining human expertise with data-driven insights, CADDIE software aids detection of high-risk lesions such as SSLs, large polyps and adenomas, as well as hard-to-detect SSLs and non-polypoid lesions.5
This capability has generated the following results per colonoscopy in a multi-center randomized controlled trial conducted across eight European hospitals:5
- 136% relative increase in the detection of large polyps (>10 mm)
- 93% relative increase in the detection of large adenomas (>10 mm)
- 57% relative increase in the detection of flat adenomas
- 230% relative increase in the detection of SSLs
Get the download on intelligent endoscopy, coupled with key Olympus® solutions for colorectal cancer screening and detection.
Simple & Scalable
CADDIE™ Software Clinical Workflow Integration
Easy to use
Hands-free interaction enabled with foot pedal.7
Simple setup
Integration with existing workflows and endoscopy equipment:8
- Olympus® CV-1500 EVIS X1™ endoscopy system9
- Olympus® CV-190 EVIS EXERA™ III endoscopy system9
The OLYSENSE Platform does not require integration with other software systems such as HIS, PACS, or EHRs.10
Cloud-based software
Secure, cloud-based software uses OLYSENSE™ Hub connected to the endoscopy tower to stream video from the procedure room to the cloud.11
Real-time analysis sends results back to endoscopy monitor for the endoscopist to review.11
Automatic updates
Remote-based updates for software.
Latest AI is automatically accessible—no onsite installation required by a service professional.
Unlock the power of intelligent endoscopy with Olympus’ OLYSENSE™ Platform and CADDIE™ software, part of the EVIS X1™ endoscopy ecosystem.
Complete the form below to receive additional information and contact us to find out more!
For detailed information regarding instructions for use, indications, contraindications, warnings, and precautions, please consult the device manual.
The CADDIE™ computer-assisted detection device is intended to assist the gastroenterologist in identifying suspected colorectal polyps only.
The gastroenterologist is responsible for reviewing CADDIE™ suspected polyp areas and confirming the presence or absence of a polyp based on their own medical judgment. The CADDIE™ device is not intended to replace a full patient evaluation, nor is it intended to be relied upon to make a primary interpretation of endoscopic procedures, medical diagnosis or recommendations of treatment/course of action for patients.
The CADDIE computer-assisted detection device is limited for use with standard white-light endoscopy imaging only.
The EVIS X1™ endoscopy system is not designed for cardiac applications. Other combinations of equipment may cause ventricular fibrillation or seriously affect the cardiac function of the patient. Improper use of endoscopes may result in patient injury, infection, bleeding, and/or perforation. Complete indications, contraindications, warnings, and cautions are available in the Instructions for Use (IFU).
- Bray Freddie, Laversanne Mathieu, Sung Hyuna, Ferlay Jacques, Siegel Rebecca L., et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA: A Cancer Journal for Clinicians, 2024 (3), 229-263 DOI: 10.3322/caac.21834.
- Zhao S, Wang S, Pan P, et al. Magnitude, Risk Factors, and Factors Associated With Adenoma Miss Rate of Tandem Colonoscopy: A Systematic Review and Meta-analysis. Gastroenterology. 2019;156(6):1661-1674.e11.
- Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370(14):1298-1306.
- ASGE.org. ASGE and ACG Release Updated Quality Indicators for Colonoscopy. Accessed August 29, 2025.
- EAGLE Trial – NCT05730192. Data on file with Odin Medical Ltd., Data not reviewed/published at time of documentation creation. Prospective randomized controlled multicenter trial sponsored by Odin Medical Ltd., a subsidiary of Olympus Corporation.
- Data on file with Odin Medical Ltd., OD-07599-BT Polyp Detection Bench Testing Report V1.4.13 Data is based on direct comparisons between use and non-use of CADDIE™ software.
- Data on file with Odin Medical Ltd, TCP-005, FDA-TCU-019.
- Data on file with Odin Medical Ltd., OD-003126-WI-6-ODIN-WI-012 — Installation instruction.
- Data on file with Odin Medical Ltd, TCU-001, TCU-003, FDA-TCU-022.
- Data on file with Odin Medical Ltd, TCU-001.
- Data on file with Odin Medical Ltd, FDA-TCU-025, FDA-TCU-026, FDA-TCU-027, FDA-TCU-028.