Your Comprehensive Portfolio of Solutions
With Olympus® product and service, you have all the tools and technology needed to provide high-quality procedures.
The Ambulatory Center Solution
Cancer screenings are the procedural core of your Center. Well-lit, in-focus, continuously sharp images matter.
Capital Equipment
Imaging. Scopes. Monitors.

EVIS X1™ Endoscopy System
The EVIS X1 endoscopy system provides a combination of diagnostic and therapeutic innovations, alongside well-established technologies, to streamline and improve endoscopic procedures.1 At its foundation, the CV-1500 Video System Center integrates an all-in-one video processor, LED light source, and modern touchscreen interface—delivering advanced imaging and observation modes in one streamlined platform.

Extended Depth of Field
(EDOF)™ Technology
Extended Depth of Field (EDOF) technology is Olympus’ latest endoscope innovation, delivering precise observation through continuous broad focus and seamless magnification. Exclusive to the EZ1500 series endoscopes, EDOF technology produces a continuously sharp, high-resolution image, reduces the need for focal adjustments, and enhances visibility—helping to make endoscopic procedures more efficient.2

EVIS X1 1100 Series Endoscopes
The EVIS X1 GIF-1100 gastrointestinal videoscope and CF-HQ1100DL/I Colonovideoscope deliver high-definition imaging and an ergonomic ErgoGrip design for comfort and control. Paired with the CV-1500 Video System Center, they offer innovative observation modes that support effective detection, diagnosis, and therapy.3,4,5
EVIS EXERA™ III 190 Series Endoscopes
Also compatible with the CV-1500 Video System Center, The EVIS EXERA III CF-HQ190L/I colonovideoscope and GIF-HQ190 gastroscope deliver advanced imaging performance with Dual Focus optics and Narrow Band Imaging™ (NBI™) technology for clear, detailed visualization. Responsive Insertion Technology (RIT) enhances handling and comfort, while improved image quality, simplified setup, and ergonomic design streamline endoscopic procedures from start to finish.
Interested in Olympus® Certified Pre-Owned (CPO) Products?
Digital AI/Solutions
Unlocking the Power of Intelligent Endoscopy
Connecting AI-driven endoscopy solutions with established Olympus® equipment, the OLYSENSE™ Platform is part of the EVIS X1™ endoscopy ecosystem of intelligent endoscopy solutions.
Cloud-based software
OLYSENSE™ Platform is a cloud-based, integrated and scalable suite of connected apps and solutions that create a sophisticated, simple, and scalable intelligent endoscopy system.
High-risk and hard-to-detect lesions
A key component of the OLYSENSE Platform is its CAD/AI portfolio, which includes the CADDIE™ medical device software that leverages cloud-based AI to aid detection of high-risk and hard-to-detect lesions.6
Single-Use Devices
Our single-use device portfolio provides the right solution for your clinical needs.
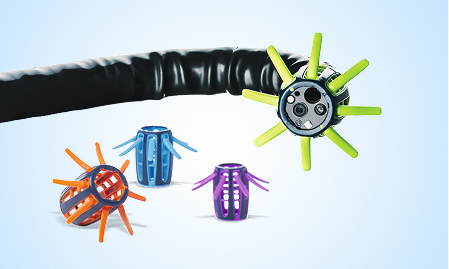
Visualize
ENDOCUFF VISION™ device, a single-use device that attaches to the distal end of a colonoscope, has been shown to increase adenoma detection rates (ADR) by manipulating colonic folds for enhanced visualization. A meta-analysis showed a 9.4% increase in ADR, with a range up to 15.1%.7
Diagnose
From biopsy to polypectomy, Olympus’® Tissue Acquisition solutions are designed for precision and efficiency. Our SnareMaster™ Plus device delivers hybrid hot and cold versatility for removal and cauterization of a variety of polyps, while EndoJaw™ forceps are designed to be used with an Olympus® endoscope to collect tissue within the digestive tract.
Treat
The intuitive design of our GI hemostasis portfolio includes Retentia™ HemoClip and EndoClot® Polysaccharide Hemostatic System (PHS) for diffuse bleeding. These technologies support both upper and lower GI procedures — helping you achieve effective hemostasis with confidence.8,9
Use consumables to unlock capital and service flexibility — smarter funding powered by the products you already use! Learn More
Infection Prevention
Clean. Compact. Intuitive.

Reprocess
The OER Elite® Endoscopic Reprocessor is designed for efficiency, simultaneously reprocesses 2 scopes in a compact 20” -wide space.

Store
ScopeLocker Air drying cabinet provides HEPA-filtered air with optional pumps and features narrow- and standard-width solutions fit for your space. It also meets AAMI ST91 2021 drying recommendations.

Transport
Identify scope status, prepare for your day’s procedures, and protect scopes during transport and storage with the Endo SafeStack transport system. Add another layer of protection during drying, transport, and storage with the PROTECH™ Flexible Endoscope Tip Protector.
Leak Testing - Keep your scopes in the procedure room and out of the service center with leak testing and endoscope protection solutions.
Service
There is a difference! Only Olympus® service can repair your equipment back to the original specifications with new or OEM certified Olympus parts. Using parts that were not designed and tested for Olympus scopes may compromise the quality of your equipment.

The OEM difference means:
- Parts, materials, and tools are all from Olympus.
- Repair processes comply with quality systems regulations.
- Rigorous reprocessing validation for compatibility with reprocessing methods and agents.

Increased Efficiencies Through Support
- Endoscopy Support Specialists
- Field Service Engineers
- Periodic Business Reviews, which can include cost containment and repair reduction efforts
- Ongoing education and support through Olympus Continuum™ Platform
Let CORE power
your center.
Reach out to speak with someone from our team to learn more about how we can support you and your Center.

For detailed information regarding instructions for use, indications, contraindications, warnings, and precautions, please consult the device manual.
The EVIS X1 endoscopy system is not designed for cardiac applications. Other combinations of equipment may cause ventricular fibrillation or seriously affect the cardiac function of the patient. Improper use of endoscopes may result in patient injury, infection, bleeding, and/or perforation. Complete indications, contraindications, warnings, and cautions are available in the Instructions for Use (IFU).
The CADDIE™ computer-assisted detection device is intended to assist the gastroenterologist in identifying suspected colorectal polyps only.
The gastroenterologist is responsible for reviewing CADDIE™ suspected polyp areas and confirming the presence or absence of a polyp based on their own medical judgment. The CADDIE™ device is not intended to replace a full patient evaluation, nor is it intended to be relied upon to make a primary interpretation of endoscopic procedures, medical diagnosis or recommendations of treatment/course of action for patients.
The CADDIE computer-assisted detection device is limited for use with standard white-light endoscopy imaging only.
Retentia HemoClip is manufactured by Yangzhou Fartley Medical Instrument Technology Co, Ltd and distributed by Olympus. EndoClot PHS is manufactured by EndoClot Plus Co., Ltd and distributed by Olympus.
Endo SafeStack transport system and Protech endoscope protector are manufactured by Meditech Endoscopy Ltd., and distributed by Olympus.
EDOF and NBI are trademarks of Olympus Corporation, Olympus America, Inc., and/or their affiliates.
NBI technology is not intended to replace histopathological sampling as a means of diagnosis.
- Data on file with Olympus (DC00303282).
- Data on file with Olympus (DC00493386, DC00433276, DC00510434 and DC00567392).
- Data on file with Olympus (DC00482729, DC00600786, DC00482729, DC00031984, DC00482967 and DC00482747)
- Data on file with Olympus (DC00661980).
- Data on file with Olympus (DC00436067).
- EAGLE Trial – NCT05730192. Data on file with Odin Medical Ltd., Data not reviewed/published at time of documentation creation. Prospective randomized controlled multicenter trial sponsored by Odin Medical Ltd., a subsidiary of Olympus Corporation.
- Patel HK, Chandrasekar VT, Srinivasan S, et al. Second-generation distal attachment cuff improves adenoma detection rate: meta-analysis of randomized controlled trials. Gastrointest Endosc. 2021; 93(3):544-553.e7.
- Data on file with Yangzhou Fartley Medical Instrument Technology Co., Ltd
- Data on file with EndoClot Plus Co., Ltd.