Olympus Selected to Exhibit iTind Device for BPH Treatment at Vizient Innovative Technology Exchange

The iTind™ device, a temporarily implanted device for relief of enlarged prostate symptoms, will be exhibited at the Vizient Innovative Technology Exchange being held virtually September 21-23.
CENTER VALLEY, Pa., (September 20, 2021) – Olympus has been selected to exhibit the iTind™ device, a temporarily implanted device for relief of enlarged prostate symptoms, at the Vizient Innovative Technology Exchange. Vizient, Inc, the nation’s largest member-driven health care performance improvement company, will hold the Exchange virtually September 21-23.
The annual Innovative Technology Exchange offers selected suppliers the unique opportunity to demonstrate their technologies to supply chain and clinical leaders from Vizient’s member hospitals and subject matter experts who serve on their supply councils. Each technology will showcase how it improves clinical outcomes, enhances safety or drives incremental improvements to health care delivery or business models.
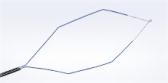
Treatment with the iTind device avoids complications associated with drugs, surgery, or permanent implants, and it allows men to go back to their normal lives shortly after the treatment.i The iTind device works by reshaping the prostate — the device’s three nitinol (nickel titanium alloy) struts gradually expand during the 5-7 days the temporary implant is in place, creating channels through which urine can flow. A U.S. multicenter study showed that the iTind provides rapid and effective relief of BPH (benign prostatic hyperplasia) symptoms while preserving sexual function.ii Placement and retrieval can be done in a hospital, ambulatory surgery center, or medical office setting.
“Olympus is thrilled to bring the iTind device to the Vizient Innovative Technology Exchange,” said Randy Clark, President of the Olympus Medical Systems Group. “This truly minimally invasive treatment gives urologists and patients a further treatment option, one that rapidly relieves enlarged prostate symptoms while preserving sexual function. We are very excited to show off the iTind device and its positive outcomes as they align with our purpose of making people’s lives healthier, safer and more fulfilling.”
“Suppliers come to the Exchange hoping to be awarded an Innovative Technology contract, which signals health care providers of their product’s unique qualities,” said Debbie Archer, procurement compliance director for Vizient. “We are pleased to include this technology in the group selected to participate.”
The annual Innovative Technology Exchange is part of Vizient’s Innovative Technology Program that includes product review of supplier-submitted technologies by member-led councils and task forces. Since 2003, Vizient has received over 2,800 technology submissions as part of its Innovative Technology Program.
# # #
About Olympus
As a leading medical technology company, Olympus uses innovative capabilities in medical technology, therapeutic intervention, and precision manufacturing to help healthcare professionals deliver diagnostic, therapeutic, and minimally invasive procedures to improve clinical outcomes, reduce overall costs, and enhance the quality of life for patients. Olympus’ Medical portfolio includes endoscopes, laparoscopes, and video imaging systems, as well as surgical energy devices, system integration solutions, medical services, and a wide range of EndoTherapy devices. For more information, visit https://medical.olympusamerica.com.
- Implantation of the iTind device may cause urinary urgency, pelvic discomfort, dysuria or hematuria. In rare cases, iTind may cause urinary tract infection or acute urinary retention.
- Chughtai B, Elterman D, Shore N, et al. The iTind Temporarily Implanted Nitinol Device for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia: A Multicenter, Randomized, Controlled Trial [published online ahead of print, 2020 Dec 26]. Urology. 2020;S0090-4295(20)31520-X. DOI: 10.1016/j.urology.2020.12.022