Spiration Valve System for Treatment of Severe Emphysema

Endobronchial Valve Treatment
Spiration Valve System for Treatment of Severe Emphysema
Emphysema is a type of chronic obstructive pulmonary disease (COPD) which is progressive in nature and characterized by a loss of elasticity and enlargement of the air sacs of the lung. As a result, the diseased lobes of the lungs become hyperinflated causing significant challenges with breathing.
A novel procedure for severe emphysema, bronchoscopic lung volume reduction (BLVR) achieved using endobronchial valves is a minimally-invasive therapy for patients who are already on optimal medical management. In the right patients, treatment with endobronchial valves can significantly improve lung function by redirecting air away from hyperinflated portions of the lung to healthier portions, allowing them to function more normally.
Based on safety and efficacy data from multiple international clinical studies, BLVR achieved using endobronchial valves is now recommended by numerous prominent guidelines as a treatment option for advanced emphysema:
- 2017 Global Initiative for Chronic Obstructive Lung Disease (GOLD)1
- National Institute for Health and Care Excellence Interventional Procedures Guidance (NICE)2
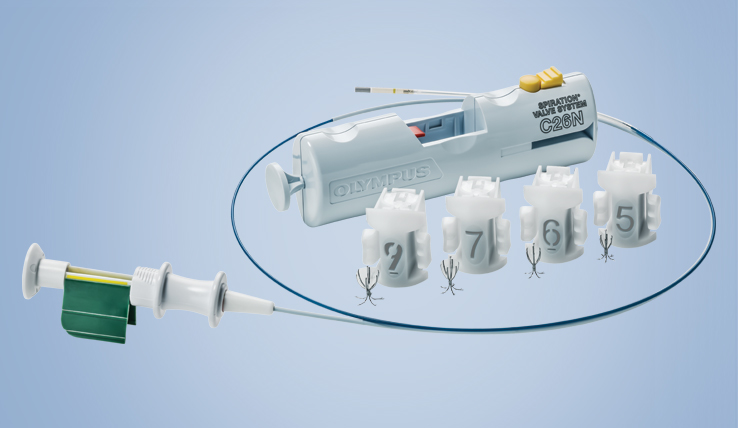
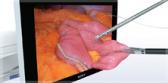
The Spiration Valve System (SVS) is an innovative endobronchial technology that offers patients with severe emphysema a customized, minimally invasive treatment option for lung volume reduction with a favorable risk-benefit profile. In clinical trials, patients treated with the SVS experienced improved breathing, lung function, and quality of life.3
For more information, click here.
Key Benefits
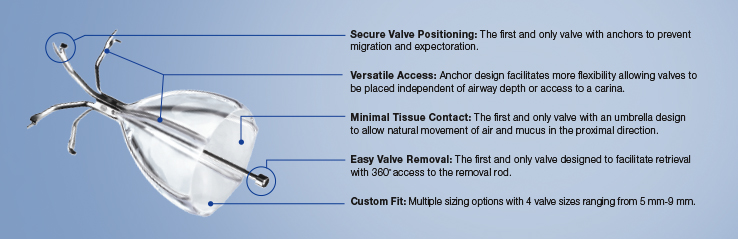
Inspired by an aerodynamic design, the Spiration Valve redirects air away from diseased areas of the lung to healthier tissue, all while allowing trapped air and secretions to escape naturally along the airway wall, so that patients may breathe easier. The unique design of the Spiration Valve allows for flexible valve placement in tortuous anatomy, such as airways in the upper lobe segments, independent of airway depth or absence of a carina.
Procedure Benefits3,4
Procedure benefits may include:
- Reduction in hyperinflation
- Improvements in pulmonary function as indicated by a change in FEV1
- Improved quality of life measured by St. George's Respiratory Questionnaire (SGRQ)
- Less dyspnea measured by mMRC
Indications for Use
Spiration Valves are one-way endobronchial valves indicated for adult patients with shortness of breath and hyperinflation associated with severe emphysema in regions of the lung that have evidence of low collateral ventilation.
Contraindications
- Patient is not an appropriate candidate for, or unable to tolerate, flexible bronchoscopy procedures.
- Patients with known or suspected sensitivity or allergy to nickel.
- Patients with evidence of active pulmonary infection.
- Patients who have not quit smoking.
- Patients with large bullae encompassing greater than 30% of either lung.
- Patients with diffuse homogenous emphysema.
General Warnings and Precautions
The following are general warnings: The safety and effectiveness of the Spiration Valve System have not been studied in
- Prior major lung or chest surgery
- Lung Volume Reduction Surgery (LVRS)
- Transplant
- Median sternotomy or thoracotomy
- Known cardiac history of myocardial infarction or congestive heart failure
- Implanted endobronchial valve currently treating a prolonged air leak
Read the complete Prescriptive Information here.
Spiration® Reimbursement Helpline
Olympus has designated services and programs available to assist you with all of your reimbursement questions and needs related to the Spiration Valve System.
Contact Information:
Hours: 9:00 am – 5:00 pm Eastern Standard Time
Phone: 855-428-7346
Email: Spirationvalvereim@olympus.com
1 2019 Global Strategy for the Diagnosis, Management, and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2018. http://goldcopd.org
2 National Institute for Health and Care Excellence (2017) Endobroncial valve insertion to reduce lung volume in emphysema (NICE guideline IPG 600) Available at: nice.org.uk/guidance/ipg600 [Accessed 16 November 2018].
3 Criner GJ, et al. 2018. The EMPROVE Trial - a Randomized, Controlled Multicenter Clinical Study to Evaluate the Safety and Effectiveness of the Spiration® Valve System for Single Lobe Treatment of Severe Emphysema. American Thoracic Society Conference. Abstract A7753.
4 Spiration Valve System. Summary of Safety and Effectiveness. 2018. Data on file.
Product Support
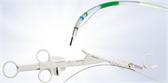
Spiration Valves
A single-use, one-way bronchial valve preloaded in a disposable cartridge.
| Catalog Number | Valve in Cartridge |
| SVS-V5-00 | 5mm |
| SVS-V6-00 | 6mm |
| SVS-V7-00 | 7mm |
| SVS-V9-00 | 9mm |
Deployment Catheter and Loader
A convenient deployment system for the delivery of multiple valves during a single patient procedure
| Catalog Number | Minimum Working Channel |
| SVS-C26N-06 | 2.6mm or greater inner diameter Deployment Catheter Length 1050mm |
Airway Sizing Kit
A kit used to determine the appropriate valve size for each target airway
| Catalog Number | Kit Includes |
| SVS-VSK-KIT | 500 microliter glass syringe with a plunger, a calibration gauge, Olympus balloon catheter (B5-2C), and a sizing worksheet |
Ancillary equipment needed for each procedure
- Flexible therapeutic bronchoscope with a working channel inner diameter of 2.6mm or greater
- Bronchoscopy forceps appropriate for valve removal
- Standard 10cc sterile syringe with Luer-lock
- Sterile saline (approximately 15-30cc used per procedure)