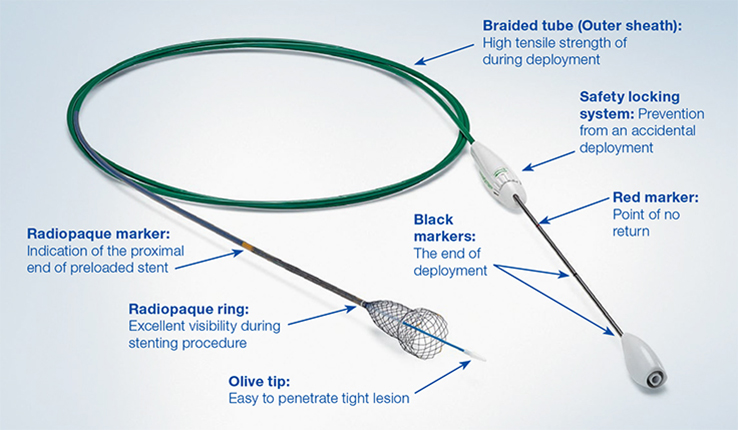
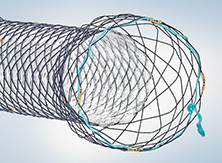
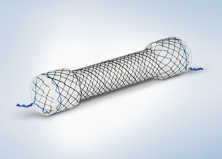
Olympus offers a lineup of self-expanding metal stents to help achieve luminal patency in a variety of clinical applications. With a unique braiding design, the HANAROSTENT® provides an ideal balance of radial and axial force, allowing for the flexibility to conform to a patient’s anatomy and precisely target their stricture.
Unlike over-the-wire esophageal stents, the 10.5F HANAROSTENT Esophagus TTS allows for accurate and easy esophageal stenting under direct scope endoscopic visualization.
HANAROSTENT® Esophagus TTS is offered in fully covered (ECBA series) or partially covered (EPBA) stent options.
Through-the-scope delivery system is compatible with a 0.035” or 0.038” guidewire.
Indications
The HANAROSTENT® Esophagus TTS (CCC) and HANAROSTENT® Esophagus TTS (NCN) are intended for maintaining esophageal luminal patency in esophageal luminal patency in esophageal strictures caused by intrinsic and/or extrinsic malignant tumors and occlusion of concurrent esophageal fistulas.
Contraindications
- Strictures that cannot be dilated enough to pass the delivery device
- Chronically bleeding tumors if bleeding is active at the time of placement
- Patients for whom the endoscopic treatments are contraindicated
- Multiple sites of obstruction
- Standard endoscopy contraindications
- Strictures caused by benign tumors
- Strictures that do not allow passage of a guidewire
- Any use other than those mentioned in the IFU (Instructions for Use)