New Olympus EU-ME2 Ultrasound Processor Delivers Versatile, Cost-Effective Resource for Sharing Across Specialties
Ultrasound Imaging Platform Supports Better Detection and Characterization of
Lesions in the Gastrointestinal Tract and Airways
CENTER VALLEY, Pa., May 2, 2014 – Olympus, a precision technology leader in designing and delivering innovative diagnostic and therapeutic solutions in Medical and Surgical procedures, among other core businesses, announced today the launch of the next-generation EU-ME2 Ultrasound Processor, which can integrate endoscopic and endobronchial ultrasound (EUS/EBUS) on a single workstation. The EU-ME2 provides better performance than current solutions and realizes cost savings through cross-departmental functionality for the GI and pulmonary markets.
GI Market/EUS
Olympus is a pioneer of endoscopic ultrasound (EUS), which combines ultrasound technology with endoscopy to better visualize the tissues of the digestive tract and adjacent anatomical structures inside the human body. With EUS, the transducer is endoscopically inserted into the body via the digestive tract, putting it closer to the area of interest to obtain higher resolution images.
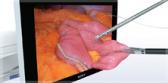
For EUS, the EU-ME2 provides outstanding image quality, comparable to a large radiology processor, in a compact model. It is designed to meet the needs of gastroenterologists performing a wide range of EUS procedures. New and enhanced features improve visualization and assist in diagnostic, therapeutic and interventional procedures for cancers and diseases of the GI track and surrounding organs, such as the pancreas, bile duct, liver, spleen and gallbladder.
“EU-ME2 is a major development in the field of pancreatic-biliary endoscopy, as EUS is increasingly being used for accessing the bile duct following a failed cannulation at ERCP,” said Dr. Shyam Varadarajulu, Medical Director of the Center for Interventional Endoscopy (CIE) and Professor of Medicine at the University of Central Florida. “The technology is advanced, compact, versatile and packed with “high-end” features; it seamlessly integrates diagnostic and therapeutic capabilities that will elevate care to the next higher level for years to come.”
According to the American Cancer Society, there are more than 125,000 newly diagnosed cases of gastrointestinal-related cancers each year in the United States with more than 91,000 deaths from these diseases in 2013. In addition, Truven Health estimates that the total number of EUS outpatient procedures will grow 15% from 2011 to 2016 and reach 235,000 procedures.[i]
Pulmonary Market/EBUS
The system also offers full support for endobronchial ultrasound (EBUS) procedures. EBUS allows visualization of the airways and adjacent structures. Along with its predecessor, Olympus’ EU-ME2 is the only platform that offers both linear and radial EBUS capabilities in one ultrasound processor for enhanced procedural and cost efficiencies.
New ACCP Lung Cancer Guidelines recommend EBUS-TBNA (transbronchial needle aspiration) over surgical staging for mediastinal staging of lung cancer as a best first test. EBUS-TBNA offers a nonsurgical solution using real-time ultrasound-guided tissue sampling. In addition, radial EBUS is recommended as an adjunct imaging modality for patients who have a peripheral lung nodule and for whom a tissue diagnosis is required due to uncertainty of diagnosis or poor surgical candidacy. EBUS-TBNA and radial EBUS support procedures for early, minimally invasive diagnosis and lung cancer staging. Both methods are beneficial for patient care and support cost-sensitive goals since each procedure can be performed on an outpatient basis instead of requiring a more complex procedure or surgery.
According to Lung Cancer Alliance data, lung cancer is the leading cause of all cancer-related deaths in the United States among every ethnic group, taking more lives than breast, prostate and colon cancers combined. Only 15% of lung cancer is diagnosed at its earliest and most curable stage, and more than 55% of cases are diagnosed after the cancer has metastasized.
EU-ME2 Benefits
The versatile EU-ME2 is forward and backward compatible with a wide range of Olympus EUS and EBUS scopes as well as ultrasound miniature probes. Since the processor is appropriate for gastroenterology, pulmonary and thoracic surgery departments, it offers healthcare facilities with a cost-effective resource that can be shared across specialties.
“As healthcare facilities look for ways to improve patient satisfaction while reducing care delivery costs, we are pleased to be able to offer our customers a versatile, state-of-the-art ultrasound platform for GI and pulmonary applications,” said Luke Calcraft, President of the Medical Systems Group at Olympus Corporation of the Americas. “The EU-ME2 enables physicians to realize clinical efficacy through premier imaging. It also helps healthcare facilities achieve efficient asset management to realize significant cost savings through lower cost of ownership.”
With its high-resolution imaging and an image display that promotes clear visualization, the EU-ME2 brings superb clarity to EUS and EBUS procedures, supporting better detection and characterization of lesions in the gastrointestinal tract and airways. This next-generation processor further expands the boundaries of endosonography with advanced features such as:
- Improved B-mode for more accurate identification of tumors and tissue properties and boundaries
- High Resolution Flow (H-Flow) mode to image small vessels around the tip of the endoscope for more precise anatomical identification
- Pulse Wave Doppler (PWD) mode to measure blood flow velocity in a specific location to help identify the target area of interest
- Elastography (ELST)[ii] mode to map tissue elasticity, which may help indicate disease and highlight areas to biopsy
- Tissue Harmonic Echo (THE)ii mode for improved resolution, improved signal-to-noise ratio, and reduced imaging artifacts
The EU-ME2 Ultrasound Processor will be showcased at Digestive Disease Week 2014 to be held on May 3-6 in Chicago, IL. Physicians are invited to visit Booth #2118 to further explore the benefits of this versatile ultrasound imaging platform.
For more information or to evaluate the EU-ME2 Ultrasound Processor, please contact your Olympus representative or call 800-848-9024.
The EU-ME2 joins a comprehensive lineup of Olympus ultrasound processors, including the ProSound F75.
About Olympus Medical Systems Group
Olympus Medical Systems Group, a division of global technology leader Olympus, develops solutions for healthcare professionals that help improve clinical outcomes, reduce overall costs and enhance quality of life for their patients. By enabling less invasive procedures, innovative diagnostic and therapeutic endoscopy, and early stage lung cancer evaluation and treatments, Olympus is transforming the future of healthcare.
For more information visit Olympus at www.medical.olympusamerica.com.
Media Contact
Michael Levey
Olympus Corporation of the Americas
484-896-5120
michael.levey@olympus.com
[i] Data for use in this study were supplied by Truven Health Analytics Inc., Ann Arbor, Michigan (“Truven Health”). Any analysis, interpretation or conclusion based on these data is solely that of the authors and Truven Health disclaims responsibility for any such analysis, interpretation or conclusion.
[ii] Only available in the Premier Plus version of the EU-ME2.