Olympus Expands VisiGlide Guidewire Lineup to Deliver Full Spectrum Solution for Endoscopies in the Biliary Tract
Newest VisiGlide 2TM Guidewire from Olympus Delivers Precise Handling for
Therapeutic Work in Sensitive Anatomy
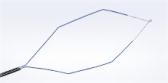
CENTER VALLEY, Pa., (October 20, 2014) – Olympus, a global technology leader in designing and delivering innovative solutions for medical and surgical procedures, among other core businesses, announced today the commercial availability of its 510(k) cleared, single-use VisiGlide 2 guidewires. Designed for use in endoscopic treatment of the biliary and pancreatic ducts, the enhanced flexibility of VisiGlide 2 tip makes this guidewire ideal for difficult cannulations and tight anatomy during biliary endoscopies.
Biliary endoscopy, or endoscopic retrograde cholangiopancreatography (ERCP), is a procedure that combines endoscopy and fluoroscopy (internal imaging to obtain real-time, moving X-ray images of internal structures). ERCP enables physicians to diagnose and treat problems in the gallbladder, bile ducts, pancreas and liver, such as gallstones, strictures, leaks resulting from trauma or surgery, and cancers. According to Truven Health Analytics data, more than 559,000 ERCP procedures were performed in the United States in 2012. [i] During ERCP, guidewires are frequently used to obtain initial access to a target duct or confined space and then help advance bulkier devices to the desired location.
"The navigational ability of the VisiGlide 2 exceeds any wire that I've used previously,” said Dr. David Diehl, Director of Interventional Endoscopy, Geisinger Medical Center, Danville, PA. “The tip can negotiate difficult strictures, and with its one-to-one torque control, I can directly steer the wire exactly where I want it to go."
Olympus VisiGlide guidewires are part of the innovative V-System, designed to enhance the success rate of ERCP. These guidewires include advanced fluoroscopic and endoscopic markings to assist with stricture measurement and a hydrophilic tip improves ductal navigation. Both 0.025- and 0.035-inch guidewires are available in 270 cm and 450 cm working lengths, with both straight and angled tips.
Coupled with the same high-performance hydrophilic coating of the standard VisiGlide, the VisiGlide 2 provides optimal tip flexibility for smooth ductal navigation, reducing risk of tissue trauma and making it an excellent choice for pancreatic anatomy. It can be used for guiding and exchanging endoscopic accessories in the biliary system, including the common bile, cystic, pancreatic and right and left hepatic ducts. The VisiGlide 2 is also safe to use during sphincterotomy.
Key features of the VisiGlide 2 include:
- Precise Control: A flexible tip paired with Terumo’s GLIDEWIRE® coating provides excellent navigation for tight ductal anatomy, such as strictures and pancreatic ducts.
- Atraumatic Design: A tapered core wire at the hydrophilic distal tip provides optimal flexibility and soft tissue contact to work well in sensitive anatomy.
- Excellent Torque: One-to-one torque control as well as a tapered hydrophilic tip aid in guidewire navigation and selective duct insertion. The angled tip version of VisiGlide 2 further enhances control during delicate procedures.
- Signature Brand Features: VisiGlide 2 maintains the key features of the VisiGlide product line, including a 0.025-inch diameter with the stiffness of a 0.035-inch guidewire, highly visible radiopaque and endoscopic markings, and proprietary coatings.
"We are very excited to launch the VisiGlide 2 ERCP guidewire to handle the complex challenges encountered during advanced biliary endoscopies,” said Rick Harbuck, Group Vice President at Olympus America Inc. “Our expanded line of VisiGlide guidewires provide physicians with the specific tools they need for therapeutic treatment in the biliary and pancreatic ducts to help ensure the best procedural outcomes. Designed with performance and patient safety in mind, these tools can help facilities address the requirements of healthcare reform aimed at improving quality of care, decreasing costs and enhancing patient satisfaction.”
Olympus VisiGlide 2 guidewires will be showcased October 19 at the ACG 2014 Annual Scientific Meeting and Postgraduate Course held in Philadelphia, Pa.
For more information about VisiGlide 2, please contact Olympus customer service at 1-800-848-9024 or visit medical.olympusamerica.com/products/guidewires/visiglide-2-guidewires.
About Olympus Medical Systems Group
Olympus Medical Systems Group, a division of global technology leader Olympus, develops solutions for healthcare professionals that help improve clinical outcomes, reduce overall costs and enhance quality of life for their patients. By enabling less invasive procedures, innovative diagnostic and therapeutic endoscopy, and early stage lung cancer evaluation and treatments, Olympus is transforming the future of healthcare.
For more information visit Olympus at medical.olympusamerica.com.
[i] Data for use in this study were supplied by Truven Health Analytics Inc., Ann Arbor, Michigan (“Truven Health”). Any analysis, interpretation or conclusion based on these data is solely that of the authors and Truven Health disclaims responsibility for any such analysis, interpretation or conclusion.
*Dr. Diehl is a paid consultant of Olympus.