Olympus Improves Specimen Sample Size for More Targeted Therapy in Lung Cancer
ViziShot FLEX 19G is the largest available EBUS-TBNA needle, potentially enabling physicians to provide more targeted therapy for lung cancer patients
CENTER VALLEY, Pa., (May 12, 2016) - Olympus, a global technology leader in designing and delivering innovative solutions for medical and surgical procedures, among other core businesses, has announced the introduction of its 510(k) cleared ViziShot FLEX 19G EBUS-TBNA Needle, used in Endobronchial Ultrasound Transbronchial Needle Aspiration (EBUS-TBNA), - a minimally invasive alternative to surgical procedures and considered the gold standard for lung cancer staging.
EBUS uses a bronschoscope equipped with ultrasound capabilities to "see" beyond the walls of the airways and detect diseased tissue, lymph nodes or lesions. Suspect lymph nodes can then be sampled using a special aspiration needle. This new needle provides a larger sample size, which can provide more material for molecular biomarker testing, potentially leading to more targeted therapy for the patient. Representing a 72 percent increase in inner surface area over the Olympus ViziShot 21G needle and 144 percent increase over the ViziShot 22G needle, the ViziShot FLEX 19g is the largest EBUS-TBNA needle on the market.
In July 2013, The U.S. Preventive Services Task Force (USPSTF) announced new recommendations for lung cancer screening for people at high risk for lung cancer, leading to increases in CT screening. Many of these patients require follow-up care that could, by USPSTF estimates, prevent roughly 14% of the 160,000 lung cancer deaths each year. Recent CMS increases in reimbursement for procedures related to lung cancer diagnosis allow hospitals to more readily build their lung cancer program. Around the same time, the American College of Chest Physicians changed its lung cancer guidelines, now recommending Endobronchial Ultrasound over surgery for lung cancer staging.
Benefits of the ViziShot FLEX 19G EBUS-TBNA needle include:
- Larger sample size: The large 19G EBUS-TBNA needle enables substantial tissue collection, even in highly challenging areas enabling genetic biomarker testing to determine the correct therapy.
- Better access during EBUS-TBNA: The ViziShot FLEX 19G provides unprecedented flexibility allowing the Olympus EBUS scope to flex up to 84°i for improved access to difficult-to-reach areas and improved sample acquisition. The echogenicity of the needle (its ability to be better seen via ultrasound) also improves precision and control.
- Safety: All of Olympus' EBUS-TBNA needles offer an advanced design with proprietary safety features including a double-locking mechanism to help avoid accidental needle protrusion protecting the patient, staff and the EBUS scope.
- A proven track record: Unlike other companies that supply EBUS-TBNA needles, Olympus has a proven track record since introducing the first ViziShot EBUS-TBNA needle to the market in 2005. Multiple clinical studies have shown a consistent diagnostic yield above 90%.
Lung cancer is the leading cause of cancer-related deaths in the U.S., according to the Centers for Disease Control, with more than 400 Americans each day dying from the disease. i Early diagnosis and accurate staging of lung cancer can lead to improved treatment. Other diseases are also better managed via EBUS-TBNA. The large specimens made possible with the ViziShot FLEX 19G may also help to improve diagnosis of lymphoma, which is cancer of the lymphatic system and sarcoidosis, an inflammatory disease affecting the lungs and lymph glands.
"This development is an important contribution to the growing field of targeted therapy and will please pulmonologists, thoracic surgeons, thoracic oncologists, and pathologists," said Kurt Heine, Group Vice President of the Endoscopy Division at Olympus America Inc. "We're excited about our team's efforts to further advance diagnosis of lung disease and lung cancer staging."
Designed with performance and patient safety in mind, Olympus' full suite of diagnostic and therapeutic devices help physicians deliver value to patients and help healthcare facilities meet the following key healthcare reform initiatives:
- Increased Quality of Care: Olympus' endoscopic platforms contribute to easier, more precise access to complicated anatomy, while maintaining the highest safety standards. The ViziShot FLEX 19G needle can yield a larger sample, which can help with targeted therapies.
- Decreased Costs: The ViziShot FLEX 19G needle may reduce the pursuit of ineffective procedures, which can be costly for little pay-off in terms of patient outcomes.
- Enhanced Patient Satisfaction: Targeted therapy can lead to better outcomes, which can lead to improved patient satisfaction.
"Today so many options exist for patients - but without a good understanding of exactly the problem we're up against, the range of solutions can create more uncertainty," said Alexander Chen, M.D., Associate Professor of Medicine. i "There is an increasing demand on bronchoscopists to provide more tissue for the purposes of biomolecular testing. The ViziShot FLEX 19g needle helps us feel more confident that we're obtaining larger specimens. I've been impressed with the early results with this needle and like all Olympus ViziShot needles it also has the double-locking safety mechanism."
The ViziShot FLEX EBUS-TBNA 19G needle will be demonstrated at the American Thoracic Society (ATS) conference to be held May 13-18 in San Francisco, Calif. Healthcare providers are invited to visit the Olympus Booth for a hands-on product demonstration. For more information about the ViziShot FLEX needle, please contact Olympus customer service at 1-800-848-9024 or visit our product page.
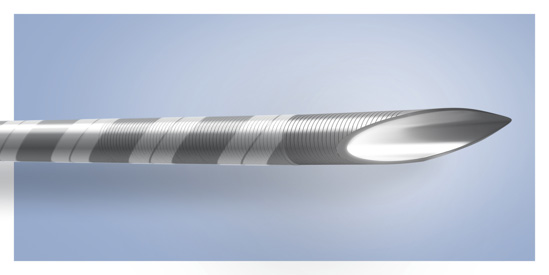
Olympus’ ViziShot FLEX 19G is the largest available EBUS-TBNA needle, potentially enabling physicians to provide more targeted therapy for lung cancer patients.
# # #
About Olympus Medical Systems Group
Olympus Medical Systems Group, a division of global technology leader Olympus, develops solutions for healthcare professionals that help improve clinical outcomes, reduce overall costs and enhance quality of life for their patients. By enabling less invasive procedures, innovative diagnostic and therapeutic endoscopy, and early stage lung cancer evaluation and treatments, Olympus is transforming the future of healthcare.
For more information visit Olympus at medical.olympusamerica.com.
i When used with the Olympus BF-UC180F 2.2mm channel EBUS bronchoscope. Data on file.
ii Centers for Disease Control and Detection: Deaths: National Vital Statistics Reports, Final Data for 2012. NVSR Volume 63, Number 9. 85 pp. (PHS) 2014 -1120, http://www.cdc.gov/nchs/data/nvsr/nvsr63/nvsr63_09.pdf
iii Dr. Chen is a paid consultant of Olympus.