FDA Clears Olympus’ Narrow Band Imaging® (NBI) for Effective Targeting of Bladder Cancer Biopsies and Improved Visualization of Tumor Margins
CENTER VALLEY, Pa., (December 15, 2014) – Olympus, a global technology leader in designing and delivering innovative solutions for medical and surgical procedures, among other core businesses, announced today FDA 510(k) clearance of Narrow Band Imaging® (NBI) as enabling effective targeting of biopsies not seen under white light and improved visualization of tumor boundaries in Non-Muscle-Invasive Bladder Cancer (NMIBC) patients.
Based on a weighted average, the aggregated FDA-reviewed studies [ii] show NBI has visualized NMIBC lesions in:
- 17 percent additional patients when compared with white light
- 24 percent additional tumors
- 28 percent additional carcinoma in situ (CIS or difficult-to-detect flat lesions)
This finding provides new treatment opportunities for urologists both in-office and in the O.R. Additionally, it offers the potential for improved cost reduction and better patient outcomes resulting from earlier detection.
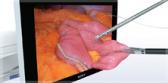
NBI is the world’s only patented endoscopic light technology that enables effective targeting of biopsies not seen under white light without the use of dyes or drugs. NBI is not intended to replace histopathological sampling as a means of diagnosis. NBI enhances visibility of vascular structures on the mucosal surface. Unlike white light, which uses all colors in the spectrum, NBI uses only blue and green. Blue and green light are strongly absorbed by blood and appear darker than normal tissue. Blue light (415nm) highlights the shallow capillaries and green light (540nm) highlights deeper veins. NBI’s potential visualization of bladder cancer symptoms has been acknowledged by the medical community, but in 2013 a meta-analysis reviewed more than 30 disparate studies on the topic, enough to submit to the FDA.
“The detection of occult lesions in patients with de novo and recurrent bladder cancer results in markedly improved outcomes,” said Daniel Canter, M.D., Vice Chairman of the Urologic Institute of Southeastern Pennsylvania and the Department of Urology of the Einstein Healthcare Network and Associate Professor at The Fox Chase Cancer Center. “An improved view into the underlying vascularity of the lining of the bladder means an improved ability to detect and treat not just the visible, obvious tumors but also the lesions that may have been missed with traditional white light cystoscopy only. This is something most urologists have known and would agree with, but it is now backed by the FDA, which should translate into improved patient care.”
Bladder cancer is the sixth most common cancer in the United States [iii], with the highest lifetime treatment costs per patient of all cancers. [iv] White light cystoscopy misses small papillary tumors or CIS at an estimated rate of 10-20 percent. [v] Fifty-four percent of patients who go undiagnosed with CIS will progress to muscle invasive disease and require bladder removal (cystectomy) and a urinary diversion or neobladder. [vi] [vii] Ninety-six percent of patients diagnosed early will survive five years later. [viii] Bladder cancer is known to recur 50 percent of the time.
Indications for Use in Office/Clinic as well as Treatment in the O.R.
NBI can be used for NMIBC in the office/clinic for cystoscopy (diagnosis) and in the O.R. or ambulatory surgical center for resection (tumor removal or Transurethral Resection of Bladder Tumor [TURBT]).
In-office, NBI provides new advantages to physicians because the most effective targeting technologies to-date could only be used in the O.R. and could only be used a single time per patient. With NBI, small tumors may be fulgurated in-office and prevent O.R. visits if visualized early enough, reducing costs associated with a costly-to-treat disease. In addition, NBI does not require the patient to take drugs or experience the discomfort of maintaining a full bladder compared with some treatments. NBI is available in both flexible and rigid endoscopes with no contra-indications. From the patient’s perspective, the equipment that uses NBI is the same as typical imaging equipment.
In the O.R., NBI can be used prior to resection to enable effective targeting of biopsies not seen under white light. During resection, NBI can be used to enhance visibility of tumor margins. By enhancing visibility of lesion boundaries, surgeons may be able to perform a more complete resection. Unlike technologies without FDA indications that strip out colors after taking the image with white light, NBI is filtered at the light source to allow application of only blue and green, for a deeper enhancement of vasculature.
The incorporation of NBI into the treatment of bladder cancer helps facilities address key requirements of healthcare reform:
- Increased Quality of Care – Improved visualization of lesions at cystoscopy can lead to earlier intervention, which may help patients avoid more invasive forms of treatment. NBI can be used at initial cystoscopy and throughout patient management and follow-up.
- Decreased Costs – NBI is integrated into the existing video platform, with no change to the office/clinic or O.R. workflow. Early diagnosis and treatment in-office may reduce the number of costly O.R. procedures. Bladder cancer currently has the highest lifetime treatment costs per patient of all cancers. [ix]
- Enhanced Patient Satisfaction – Patients whose physicians use NBI may experience reduced treatment discomfort during treatment and avoid more invasive forms of the disease that lead to bladder removal and creation of a neo-bladder.
“We are excited to offer NBI to the urologic community and share this new development with primary care physicians who can refer patients to urologists using NBI,” said Richard Reynolds, Executive Vice President – Sales, Marketing & Shared Services of the Medical Systems Group at Olympus Corporation of the Americas. “We believe that NBI has the potential to revolutionize how bladder cancer is detected and treated, helping our customers meet the triple aim of healthcare reform by improving quality of care, decreasing costs and enhancing patient satisfaction.”
In addition to urology, NBI has clinical applications throughout the anatomy including gastroenterology, pulmonary and rhinolaryngology (ENT). Olympus has received FDA-clearance for the screening and surveillance of Barrett’s esophagus and is currently exploring other claims for NBI both in gynecology and general surgery.
Olympus has developed an NBI urology app for physicians, available in the App Store with the “Olympus NBI” search terms. The app includes 28 clinical case images, a technology overview, comparison shots of white light and NBI, with an interactive slider and the option to email a case summary to a consulting physician, staff member or patient. To learn more about NBI, visit: medical.olympusamerica.com/nbi-urology.
For more information or to evaluate NBI, please contact your Olympus representative or call 800-848-9024.
About Olympus Medical Systems Group
Olympus Medical Systems Group, a division of global technology leader Olympus, develops solutions for healthcare professionals that help improve clinical outcomes, reduce overall costs and enhance quality of life for their patients. By enabling less invasive procedures, innovative diagnostic and therapeutic endoscopy, and early stage lung cancer evaluation and treatments, Olympus is transforming the future of healthcare.
For more information, visit Olympus at medical.olympusamerica.com.
i Bladder Cancer Statistics. American Bladder Cancer Society. Dalton, MA. http://bladdercancersupport.org/component/content/article/88-accessible-pages/308-bladder-cancer-statistics.html
ii Li, K., Lin, T., Fan, X., Duan, Y., & Huang, J. (2013). Diagnosis of narrow-band imaging in non-muscle-invasive bladder cancer: A systematic review and meta-analysis. International Journal of Urology, 20, 602-609. http://www.ncbi.nlm.nih.gov/pubmed/23113702
iii SEER Cancer Statistics Factsheets: Bladder Cancer. National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/statfacts/html/urinb.html
iv Sievert, K., Amend, B., Nagele, U., Schilling, D., Bedke, J., Horstmann, M., Stenzl, A. (Jun 2009). Economic aspects of bladder cancer: What are the benefits and costs? World Journal of Urology, 27(3), 295-300. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2694315/
v Jichlinski, P., & Leisinger, H. (2005). Fluorescence Cystoscopy in the Management of Bladder Cancer: A Help for the Urologist! Urologia Internationalis, 74(2), 97-101. http://www.ncbi.nlm.nih.gov/pubmed/15756058
vi Sylvester, R., Meijden, A., Witjes, J., Jakse, G., Nonomura, N., Cheng, C. Kurth, K. (2005). High-grade Ta urothelial carcinoma and carcinoma in situ of the bladder. Urology, 66: 90–107 (Originally reference 29 of Li meta analysis). http://www.ncbi.nlm.nih.gov/pubmed/16399418
vii Li, K., Lin, T., Fan, X., Duan, Y., & Huang, J. (2013). Diagnosis of narrow-band imaging in non-muscle-invasive bladder cancer: A systematic review and meta-analysis. International Journal of Urology, 20, 602-609. http://www.ncbi.nlm.nih.gov/pubmed/23113702
viii SEER Cancer Statistics Factsheets: Bladder Cancer. National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/statfacts/html/urinb.html
ix Sievert, K., Amend, B., Nagele, U., Schilling, D., Bedke, J., Horstmann, M., Stenzl, A. (Jun 2009). Economic aspects of bladder cancer: What are the benefits and costs? World Journal of Urology, 27(3), 295-300. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2694315/
* Dr. Canter is a paid consultant of Olympus.