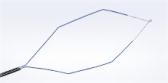
Olympus Takes Plasma Vaporization to a New Level of Efficiency with Plasma-OvalButton
Device offers enhanced patient safety through less bleeding and increased procedural efficiencies
through new design
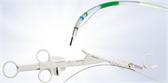
CENTER VALLEY, Pa., (May 4, 2016) – Olympus, a global technology leader in designing and delivering innovative solutions for medical and surgical procedures, among other core businesses, announced today the launch of its FDA cleared Plasma-OvalButton for urologic procedures including minimally invasive surgery to reduce enlarged prostate as well as treatment of bladder cancer. The Plasma-OvalButton uses Olympus’ plasma technology in a revolutionary electrode shape designed to maximize O.R. efficiency and enhance clinical outcomes.
With a 25 percent increase in width and 31 percent increase in vaporization area from Olympus’ present vaporization device, the Plasma-OvalButton is designed to increase tissue removal rates for enhanced procedural efficiency. Lab testing has demonstrated a 21% increase in tissue vaporization from Olympus’ existing vaporization electrode. The Plasma-OvalButton is the latest innovation launched to complement Olympus’ full line of PlasmaLoops and electrodes that provide a comprehensive Transurethral Resection (TUR) solution utilizing Olympus’ plasma technology.
Plasma vaporization therapy with the Plasma-OvalButton is a minimally invasive surgical procedure performed in saline, which has demonstrated reduced risk of TUR syndrome when compared to more traditional monopolar procedures, and is recommended by the National Institute of Health and Clinical Excellence. Other benefits of plasma vaporization therapy over monopolar resection include less bleeding, improved visualization, more precise cutting and coagulation, and shorter catheterization and procedure times.
Enlarged prostate, or benign prostatic hyperplasia (BPH), affects approximately 60 percent of men age 60 or over. The percentage increases to 80-90 percent in men over age 80. Approximately 150,000 men have transurethral resection of the prostate (TURP) procedures each year in the U.S. During the procedure, the surgeon uses an endoscopic image to guide the electrode through the urethra to the prostate to safely and efficiently remove prostatic tissue to allow the patient to return to normal urinary function. Olympus’ plasma portfolio of electrodes can also be used during TURBT – a surgical procedure to remove cancerous tissue from the bladder. Bladder cancer is the sixth most common cancer in the United States, but 96 percent of patients diagnosed early will survive five years later.
“The new Olympus Plasma-OvalButton is a tremendous advancement in the treatment of BPH,” said Dr. Kenneth Kernen, MD, Beaumont Health System. “It is more effective and will allow more TURs to be performed on an outpatient basis.”
“Olympus is pleased to strengthen our industry leading urology portfolio even further with the Plasma-OvalButton,” said Todd Usen, President, Olympus Medical Systems Group at Olympus Corporation of the Americas. “We expect that this plasma vaporization technology, already embraced by the urology community, will soon become the industry standard for how transurethral resection is conducted.”
Designed with performance and patient safety in mind, Olympus’ full suite of therapeutic devices help physicians deliver value to patients and help healthcare facilities meet the following key healthcare reform initiatives:
- Increased Quality of Care: Olympus’ plasma products reduce the risk of TUR syndrome, decrease risk of hyponatremia, and show reduction in procedural complications such as bleeding and increased catheterization time.
- Decreased Costs: Olympus’ plasma portfolio can reduce costly procedure time, through the benefits of concurrent vaporization and hemostasis as well as increased vaporization area from the current vaporization electrode.
- Enhanced Patient Satisfaction: Reduced catheterization time, reduced complications and faster recovery time are benefits that can lead to increased patient satisfaction.
The Plasma-OvalButton will be showcased at the American Urological Association’s annual meeting to be held on May 6-10 in San Diego, Calif. Healthcare providers are invited to visit the Olympus Booth #3833 for a hands-on product demonstration. For more information about the new Plasma-OvalButton solution, please contact Olympus customer service at 1-800-848-9024 or visit http://medical.olympusamerica.com/products/plasma-ovalbutton.
# # #
About Olympus Medical Systems Group
Olympus Medical Systems Group, a division of global technology leader Olympus, develops solutions for healthcare professionals that help improve clinical outcomes, reduce overall costs and enhance quality of life for their patients. By enabling less invasive procedures, innovative diagnostic and therapeutic endoscopy, and early stage lung cancer evaluation and treatments, Olympus is transforming the future of healthcare.
For more information, visit Olympus at medical.olympusamerica.com.

Olympus takes plasma vaporization to a new level of efficiency with Plasma-OvalButton

Olympus announced today the launch of its FDA cleared Plasma-OvalButton for urologic procedures including minimally invasive surgery to reduce enlarged prostate as well as the treatment of bladder cancer. With a 25 percent increase in width and 31 percent increase in vaporization area from Olympus’ present vaporization device, the Plasma-OvalButton is designed to increase tissue removal rates for enhanced procedural efficiency.
i http://www.urologyhealth.org/urologic-conditions/benign-prostatic-hyperplasia-(bph)/treatment/surgery
ii Dr. Kernen is a paid consultant of Olympus